REMAIN IN CONTROL OF YOUR LIFE
You may not have heard of ‘Allopregnanolone’, but you could well care for one of the millions who suffer from its effects.
From Tourette syndrome to OCD, ADHD, compulsive gambling, PMDD and essential tremor — the potent neurosteroid Allopregnanolone is implicated in them all.
Our novel treatment Sepranolone is the endogenous compound that modulates the effects of Allopregnanolone. It has achieved an excellent safety profile: Up to 350 women and men have taken Sepranolone, in 3 major clinical studies, with no other side effects than mild and reversible local skin irritation.
Sepranolone has been developed from over 40 years’ neuroendocrinological research.
SEPRANOLONE & MENSTRUAL MIGRAINE
POSITIVE PHASE II A RESULTS FOR SEPRANOLONE IN TOURETTE SYNDROME
“I can easily see Sepranolone becoming the new first-line treatment for Tourette patients who require pharmaceutical treatment. I believe Sepranolone has a strong future.”
Consultant neurologist Dr Heidi Biernat Bispebjerg University Hospital CopenhagenRead about the results here.
On April 1, 2023 Asarina Pharma released positive results for its Phase IIa clinical study of Sepranolone in Tourette Syndrome. Clinical Neurologist Dr Heidi Biernat, Head of the Tourette Syndrome Clinic at Bispebjerg University Hospital in Copenhagen, ran the Study. Read her thoughts on the challenges of today’s TS treatments, the patient response to Sepranolone and how she sees its potential as a first-choice treatment for pharmaceutical intervention in Tourette Syndrome.


SEPRANOLONE
“I believe Sepranolone will always attract interest from clinicians and patients, whatever indication it is launched for.”
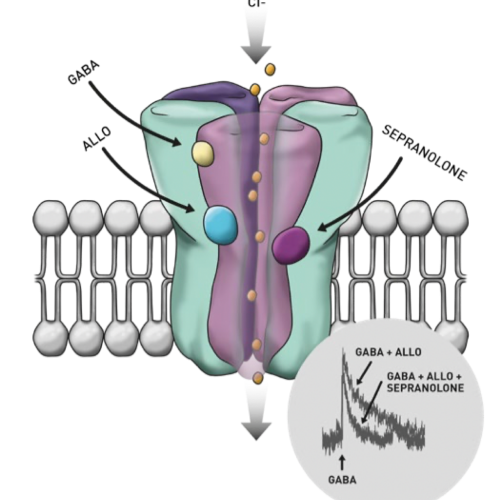
Professor Marie Bixo, University of UmeåThe neurochemical GABA is the brain’s most powerful inhibitory neurotransmitter. It plays a crucial role in inhibiting and reducing stress, fear and anxiety levels. But what keeps GABA in check? The neurosteroid Allopregnanolone (or ALLO) is a potent modulator of GABA, acting on the GABA-A receptor, a major pathway for GABA within the brain.
The body’s endogenous compound—Sepranolone—regulates and modulates the negative effects of ALLO. Asarina Pharma is the first company to have developed and synthesized Sepranolone as a medication, patenting a pharmaceutical formulation in 2010.
Sepranolone has achieved an excellent safety profile in substantial clinical trials. It was tested in a Phase IIa Tourette syndrome study in 2022, with topline results expected at the end of March 2023.
TOURETTE SYNDROME
“I believe we are on the crest of a new wave of understanding of just how broad the impact of Allopregnanolone really is. Compulsivity impacts on so many different conditions, from ADHD and OCD/B through to eating disorders and addiction.”
Assoc prof Marco Bortolato, University of Utah
Tourette syndrome is a cruel condition typically striking first between the ages of 3 – 9 years old. Yet many current treatments, like the anti-psychotic Haldol, have severe side effects ranging from blurred vision, nausea and diarrhoea to involuntary movement disorder, irregular heartbeat and even renal failure.
Sepranolone: A safer approach
In May 2019 Asarina Pharma demonstrated in a preclinical animal study that Sepranolone reduced tics on a par with Haldol, without inducing any motor side effects.
Asarina Pharma CEO Peter Nordkild: “This is a new approach, and a safe one. There are no neurosteroid-based medications currently being used to treat TS. A positive result would be extremely promising for patients, and indicate that Sepranolone could potentially play a part in treating a range of other ALLO-related stress disorders.”
OBSESSIVE-COMPULSIVE DISORDER
“OCD ‘turns dust into dynamite’ they say, it’s true for me. Even though I tell myself ‘this isn’t me, it’s the OCD’, the heaviness, the panic, can be totally overwhelming”
‘Helena’ 24, Sweden.
OCD strikes as many as 12 in every 1,000 people (1.2% of the population), with slightly more women than men affected. The impact can be devastating – with a disproportionately high number of OCD cases, about 50%, classified as severe, and less than a quarter classed as mild (OCD UK).
Lack of pharmaceutical treatment options
Yet despite its prevalence and impact, treatment options, particularly pharmaceutical ones, leave many needs unmet. ERP (exposure and response therapy), today’s front-line treatment, can work well, but is demandingand time-consuming. Relapse is common. Meanwhile, SSRIs, today’s most commonly prescribed pharma treatment, have an overall efficacy of < 50%.
Asarina Pharma’s safe, endogenous compound Sepranolone represents a new approach to treating OCD.