SEPRANOLONE AND PMDD
Professor Torbjörn Bäckström, Founder and Chief Science Officer, Asarina Pharma and Senior Professor in Obstetrics and Gynecology, University of Umeå
WHAT CAUSES PMDD?
PMDD is a caused by an altered sensitivity in the brain to the potent neurosteroid Allopregnanolone (ALLO). In the luteal phase, the two weeks leading up to a period, the ovaries start producing increased amounts of progesterone, which is then turned into ALLO in the liver.
ALLO is one of the most potent modulators of the brain’s powerful neurochemical GABA – itself the Central Nervous System’s major inhibitory neurotransmitter. ALLO acts on the brain’s GABA-A receptor, directly impacting a range of menstrual-related conditions, like PMDD, as well as neurological ones.
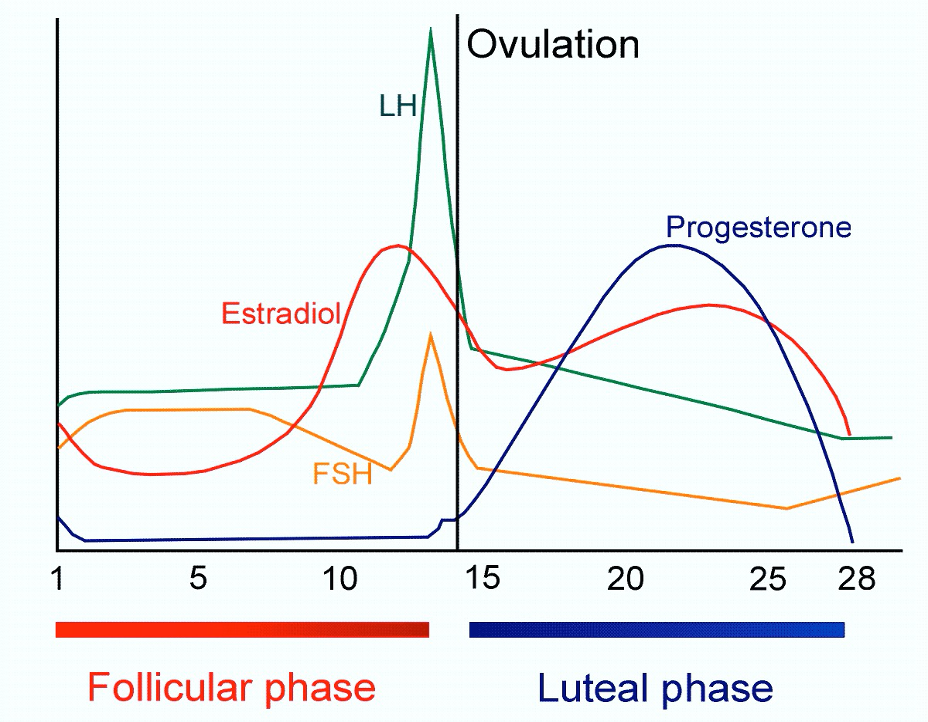
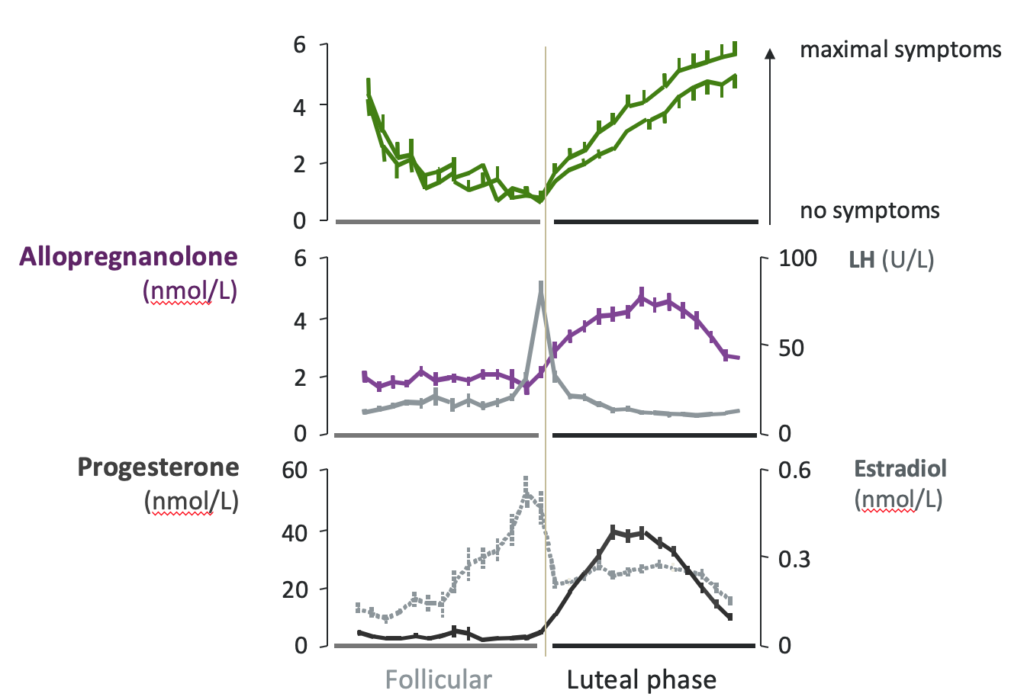
ALLO heavily influences GABA-A receptors in the brain’s emotional center, the Amygdala, impacting mood, personality and behavior . For a minority of women with a heightened sensitivity to ALLO, the results are the powerful negative symptoms of PMDD, including cardinal, or primary, emotional symptoms, secondary symptoms, and even physical symptoms.
Cardinal symptoms
- Depression
- Anxiety
- Lability
- Anger / irritability
Secondary symptoms
- Interest
- Concentration
- Appetite
- Sleep
- Overwhelmed
Physical symptoms
- Breast tenderness
- Abdominal bloating
- Headache
- Joint or muscle pain
- Exhaustion
ASARINA PHARMA AND PMDD
SEPRANOLONE is the body’s natural, endogenous, compound that modulates Allopregnanolone levels, so reducing the negative effects of ALLO. Asarina Pharma is the first company in the world to develop Sepranolone as a medication, patenting a pharmaceutical formulation of Sepranolone in 2010.
PMDD was Asarina Pharma’s first lead indication. The company developed Sepranolone, the first therapy specifically developed to treat PMDD, based on the 40 years’ research work of its founder and CSO Professor Torbjörn Bäckström, Professor in Obstetrics and Gynecology at the3 Department of Clinical Sciences University of. Umeå. The company ran two clinical studies in PMDD, a Phase IIa study in 2013 in Sweden, and a Phase IIb study in Sweden, the UK, Poland and Germany in 2018 – 2019.
PHASE IIa RESULTS: POSITIVE
The company carried out a Phase IIa exploratory clinical study in 2013.
The study was a double-blind, randomised, placebo-controlled study of 120 women in Sweden with verified PMDD.
The primary endpoint was a change in symptoms and impact of symptoms assessed by the validated rating scale for PMDD – the DRSP (Daily Record of Severity of Problem).
The study found Sepranolone reduced the key symptoms of PMDD by almost 80% compared to the placebo effect.
PHASE IIb RESULTS: INCONCLUSIVE
The company carried out a Phase IIb clinical study in 2018, reporting top-line results in April 2020.
The study was a double-blind, randomised, placebo-controlled study of 206 women in the UK, Sweden, Poland and Germany.
The active Sepranolone substance performed on a par with the previous study, with a similar reduction of both primary and secondary endpoint symptoms. However, the placebo response was 33% higher than in the Phase IIa study, meaning that no statistical difference between the treatment groups could be demonstrate and the study results were therefore inconclusive.